Standards should be applied in the prevention and handling of missing data for patient-centered outcomes research: a systematic review and expert consensus - Journal of Clinical Epidemiology

A hybrid approach of handling missing data under different missing data mechanisms: VISIBLE 1 and VARSITY trials for ulcerative colitis - ScienceDirect

Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: A practical guide - Cro - 2020 - Statistics in Medicine - Wiley Online Library

Quality of missing data reporting and handling in palliative care trials demonstrates that further development of the CONSORT statement is required: a systematic review - Journal of Clinical Epidemiology

Missing data in clinical trials: from clinical assumptions to statistical analysis using pattern mixture models - Ratitch - 2013 - Pharmaceutical Statistics - Wiley Online Library

An Empirical Comparison of Statistical Methods for Missing Data in Randomized, Double-Blind, Placebo-Controlled, Phase 3 Clinical Trials for Chronic Pain and Lipid-Lowering Products | SpringerLink
A narrative review of estimands in drug development and regulatory evaluation: old wine in new barrels? | Trials | Full Text

Biases in study design, implementation, and data analysis that distort the appraisal of clinical benefit and ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) scoring - ESMO Open
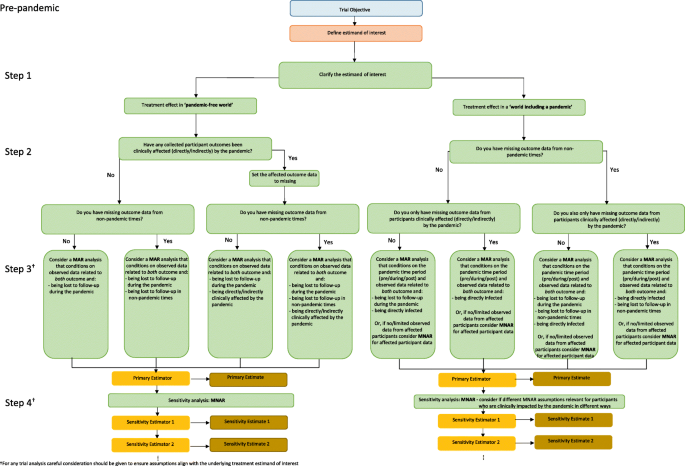
A four-step strategy for handling missing outcome data in randomised trials affected by a pandemic | BMC Medical Research Methodology | Full Text

On Biostatistics and Clinical Trials: Single Imputation Methods for Missing Data: LOCF, BOCF, LRCF (Last Rank Carried Forward), and NOCB (Next Observation Carried Backward)

Missing data in trial‐based cost‐effectiveness analysis: An incomplete journey - Leurent - 2018 - Health Economics - Wiley Online Library
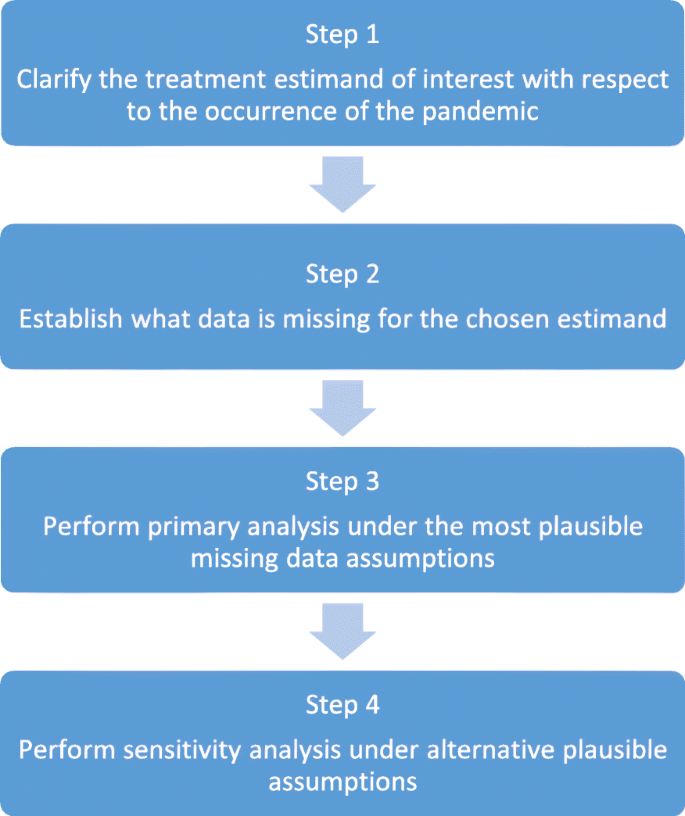
A four-step strategy for handling missing outcome data in randomised trials affected by a pandemic | BMC Medical Research Methodology | Full Text

Quality of missing data reporting and handling in palliative care trials demonstrates that further development of the CONSORT statement is required: a systematic review - ScienceDirect

PDF) The Prevention and Treatment of Missing Data in Clinical Trials | Texila International Journal - Academia.edu

Guideline on Missing Data in Confirmatory Clinical Trials / guideline-on- missing-data-in-confirmatory-clinical-trials.pdf / PDF4PRO

Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: A practical guide - Cro - 2020 - Statistics in Medicine - Wiley Online Library
Full article: Statistical Issues and Recommendations for Clinical Trials Conducted During the COVID-19 Pandemic

Considerations to address missing data when deriving clinical trial endpoints from digital health technologies - ScienceDirect

Empirical evaluation of the implementation of the EMA guideline on missing data in confirmatory clinical trials: Specification of mixed models for longitudinal data in study protocols - Häckl - 2019 - Pharmaceutical






![PDF] The prevention and treatment of missing data in clinical trials. | Semantic Scholar PDF] The prevention and treatment of missing data in clinical trials. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b5dea335239682010fe99c882ea8fb917de880b5/3-Table2-1.png)